Decontamination Area
Home / Solution
CSSD (central sterile supply department), which is responsible for the cleaning, disinfection, and sterilization of all the reused diagnostic and treatment instruments, utensils, and articles from all departments in the hospital, is also in charge of the supply of the sterile items. It is the key department for preventing and controlling hospital infections to ensure the quality of medical care. The quality management of CSSD affects medical safety and patients' lives.
JBlMED is committed to providing CSSD one-stop services such as overall design, engineering construction, and equipment supply. We will strictly follow the new norms and standards of the Ministry of Health, and wholeheartedly provide you with solution.
-
Function

-
Service

-
Professional

-
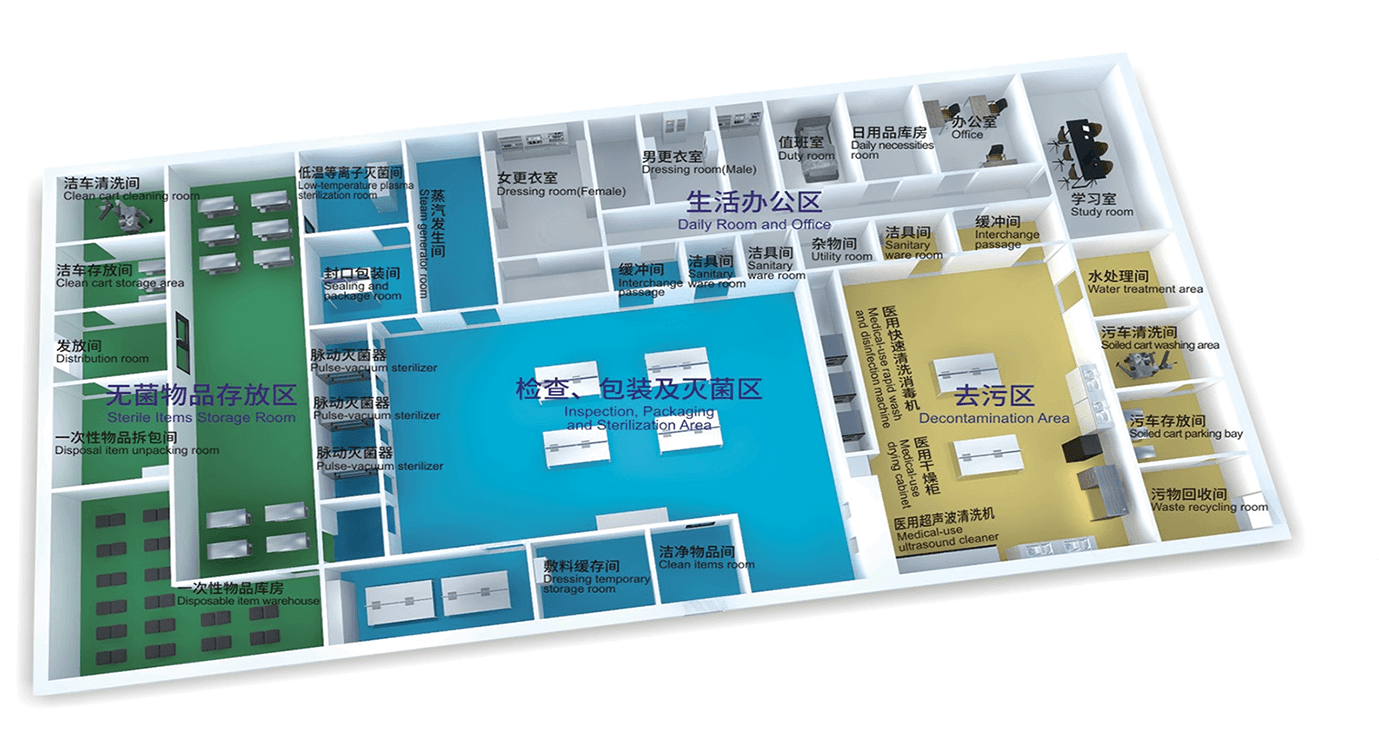
Zone partition

-
-
Inspection, Packaging and Sterilization Area
-
Sterile ltems Storage Room
-
Daily Room and Office
Design Basis
1. CSSD(Central Sterile Supply Department) Management Standard WS 310.1-2016
2. CSSD (Central Sterile Supply Department) Cleaning, Disinfection, and Sterilization Technical Operation Code WS 310.2-2016
3. CSSD(Central Sterile Supply Department) Cleaning Disinfection and Sterilization Result Test Code WS 310.3-2016
4. The Central Air Conditioning Ventilation System of Public Places Meets the Cleanliness Standard WS 394-2012
5. Technical Standard for Disinfection of MedicalInstitutions (2023 edition)
6. Disinfection Management Measures (Issued on December 26, 2017)
7. General Hospital building design standard 51039-2014
8. Standard Design Code of Clean WorkshopGB 50073-2013
9. China Hospital Construction Guidelines (2018 Edition)
10. Standard for Architectural Lighting Design (GB 50034-2013)
2. CSSD (Central Sterile Supply Department) Cleaning, Disinfection, and Sterilization Technical Operation Code WS 310.2-2016
3. CSSD(Central Sterile Supply Department) Cleaning Disinfection and Sterilization Result Test Code WS 310.3-2016
4. The Central Air Conditioning Ventilation System of Public Places Meets the Cleanliness Standard WS 394-2012
5. Technical Standard for Disinfection of MedicalInstitutions (2023 edition)
6. Disinfection Management Measures (Issued on December 26, 2017)
7. General Hospital building design standard 51039-2014
8. Standard Design Code of Clean WorkshopGB 50073-2013
9. China Hospital Construction Guidelines (2018 Edition)
10. Standard for Architectural Lighting Design (GB 50034-2013)
Design Principle
1. The whole process from a contaminated area to a clean area, without any cross or reverse.
2. Physical barriers are placed between the decontamination area, package inspection and sterilization area, and sterile area.
3. Delivery paths are established between the decontamination area and the package inspection &sterilization area; the buffer rooms should be placed separately for people to enter.
4. The handwashing facility in the buffer room should be inductive.
5. A sealing-type sanitary ware room should be included in the package inspection &sterilization area.
2. Physical barriers are placed between the decontamination area, package inspection and sterilization area, and sterile area.
3. Delivery paths are established between the decontamination area and the package inspection &sterilization area; the buffer rooms should be placed separately for people to enter.
4. The handwashing facility in the buffer room should be inductive.
5. A sealing-type sanitary ware room should be included in the package inspection &sterilization area.
Design Concept
The central sterile supply department (CSSD) plays an important role for the general hospital in providing a safe production process, safe products and the protection of medical staff of the safe environment.
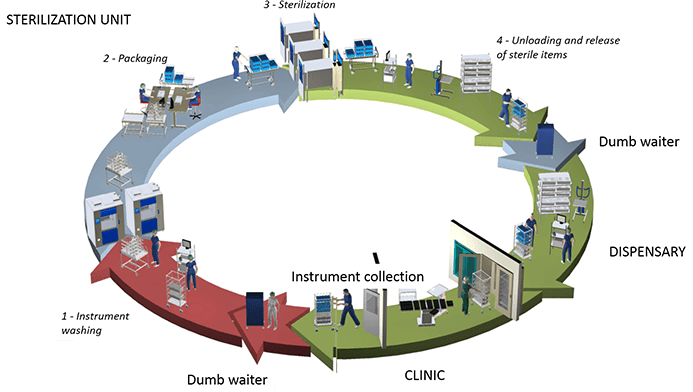
The First Shield: The first shield is located in a contaminated and clean area to prevent pathogenic microorganisms. High-throughput cleaning and sterilizing were performed by the washing-disinfector and the double-door design, which efficiently reduced cross-contamination.
The Second Shield: The second shield is located in a clean area and sterile item storage area to prevent pathogenic microorganisms.
The big double-door sterilizer functions to sterilize the cleaned and disinfected items, which ensures the safety of the sterilized items.
The First Shield: The first shield is located in a contaminated and clean area to prevent pathogenic microorganisms. High-throughput cleaning and sterilizing were performed by the washing-disinfector and the double-door design, which efficiently reduced cross-contamination.
The Second Shield: The second shield is located in a clean area and sterile item storage area to prevent pathogenic microorganisms.
The big double-door sterilizer functions to sterilize the cleaned and disinfected items, which ensures the safety of the sterilized items.
-
Personnel safety
Hazard items pre-treatment, machinery cleaning and disinfection, personnel career safety protection -
Items safety
Control the contamination source, cut off the broadcasting path, and provide protection -
Environment safety
Separately partition areas, contaminated air exhausting and treatment, airflow control
The Disinfection Supply Center Management Traceability System is a series of software products developed by Binjiang Medical based on years of experience in hospital infection control and combined with digital information technology, which not only meets the urgent need for improving CSSD management in Chinese hospitals but is also tailor-made for China's Ministry of Health's latest standards. It focuses on providing a comprehensive management solution for sterile supply in hospitals and is committed to promoting the development of hospital infection prevention and control, thereby minimizing the risk of infections.
-
 Sterilize
Sterilize  Support the empty sterilizing and daily sterilizing (high temperature or low temperature), real-time recording the sterilizing time, sterilizing staff, and the instrument packages. Provide the sterilizer statue inquiry (spare time, sterilizing standby, in sterilization), as well as displaying the detailed items information and the total amount.
Support the empty sterilizing and daily sterilizing (high temperature or low temperature), real-time recording the sterilizing time, sterilizing staff, and the instrument packages. Provide the sterilizer statue inquiry (spare time, sterilizing standby, in sterilization), as well as displaying the detailed items information and the total amount. -
 Storage
Storage  Support the empty sterilizing and the daily sterilization, real time recording the sterilizing time, sterilizing staff and the instrument packages. Provide the statues inquiry, display the detailed items information and the total amount.
Support the empty sterilizing and the daily sterilization, real time recording the sterilizing time, sterilizing staff and the instrument packages. Provide the statues inquiry, display the detailed items information and the total amount. -
 Distribution
Distribution  Support the application of the distribution of the disposable items. Support the application of the distribution of the non-disposable items. Distribution application, lend distribution, partialitems distribution. Support the distribution of the self-prepared items. Support the distribution of the surgical instruments
Support the application of the distribution of the disposable items. Support the application of the distribution of the non-disposable items. Distribution application, lend distribution, partialitems distribution. Support the distribution of the self-prepared items. Support the distribution of the surgical instruments -
 Retrieval
Retrieval  Disposable ltems Retrieval
Disposable ltems Retrieval
Retrieve the distribution of the disposable items and the related historical info, and proceed with the statistics inquiry according to the producing batch number.
Non-disposable ltems Retrieval
Fully trace all information throughout the process of device recycling, cleaning and disinfection, packaging, sterilization, distribution, and utilization. Tracing the department where the items cleaned in the same batch are located.Tracing the department where the items sterilized in the same batch are located -
 Recycling
Recycling  Support the manual recycling: input the recycling department the items.
Support the manual recycling: input the recycling department the items.
Support centralized recycling: automatically retrieve all departmental application forms.
Detailed record the recycling staff, time, recycling department and the items quantity. Recycling inquiry. -
 Cleaning
Cleaning  Record the entire process of the washing disinfector.
Record the entire process of the washing disinfector.
Cleaning procedure, staff, cleaning batch, the start and the end time.
Support the record of the manual cleaning process, including clean staff and time.
Check the cleanliness status and tray status, as well as the verification. -
 Package
Package  Support printing the barcode label on the washed items.
Support printing the barcode label on the washed items.
Support printing the barcode label on the dressing items.
Support printing the barcode label on the normal monitoring packages.Barcode identification of the package, including items name and specification, package staff, sterilizing mode, sterilizing date, expiration date, record print staff, packaging date.


 English
English русский
русский Français
Français Español
Español bahasa Indonesia
bahasa Indonesia Deutsch
Deutsch عربى
عربى 中文简体
中文简体





